In some circumstances as part of ART treatment, it is appropriate to investigate the genetic make-up of an early embryo to maximise the chances of a healthy baby being born. This is achieved by sampling, or biopsying, one or more cells from the embryo and testing those cells for a particular genetic condition. This is a specialised type of ART treatment and is called preimplantation genetic diagnosis, or PGD.
To access PGD, patients must first have a cycle of egg collection, followed by fertilisation of those eggs to form embryos. Embryo biopsy is performed during the first few days of embryo development. The embryos are maintained in culture or can be frozen while the cells are being tested.
Who should have PGD?
There are four broad indications where PGD may be an option for patient care.
These are:
1. PGD for infertility
All human cells contain dense strands of genetic material, or DNA, arranged in chromosomes. There are 24 different human chromosomes, numbered 1-22 plus X and Y. Normal cells contain two copies of each of chromosomes 1-22 and either two copies of the X-chromosome (females) or an X and a Y chromosome (males) for a total of 46 chromosomes in each cell. The only exceptions to this are sperm and egg cells which have one set of 23 chromosomes each.
Cells that have the wrong number of chromosomes are said to be aneuploid. Aneuploidy in embryos is almost always a lethal condition, with embryos often surviving only a matter of days. Trisomy 21, or Down syndrome, is one of the few examples of an aneuploidy that can result in a live birth.
A large body of research accumulated over recent years has demonstrated that aneuploidy is very common in early human embryos, with at least 50 per cent having a chromosome error. It is thought that embryos from some couples may be predisposed to aneuploidy, resulting in difficulties in achieving or maintaining pregnancy and unsuccessful ART cycles. Consequently, one of the most common applications of PGD is for couples who are experiencing infertility.
This will include couples:
- Where the woman is older, often over the age of 38 years
- Who have not achieved a successful pregnancy, despite having of a number of good quality embryos transferred in ART cycles
- Who have experienced repeated miscarriages
- Who have had a pregnancy with a chromosome abnormality, such as Down syndrome
It is thought that couples who fall into the above categories are predisposed to chromosome errors in their embryos and that PGD can assist them by identifying embryos that are free of these abnormalities, and so more likely to result in a successful pregnancy. This application of PGD is sometimes called preimplantation genetic screening, or PGS.
2. PGD for chromosome rearrangements
Some people carry a chromosomal translocation or rearrangement. This is when pieces of the person’s chromosomes are in the wrong order. For example, pieces of different chromosomes may break off and translocate, or swap places. Alternatively a piece of a chromosome may have flipped around to form an inversion. The person who carries the rearrangement is usually unaffected as they have the correct amount of DNA, it is just shuffled around. This is called a balanced translocation or rearrangement. However, carriers of translocations are at high risk of forming embryos with additional or missing pieces of chromosomes, called an unbalanced translocation or rearrangement. This can cause infertility, miscarriage or sometimes the birth of a child with significant disability.
Couples where one partner carries a chromosome rearrangement can utilise PGD to identify embryos that have a balanced complement of chromosomes.
3. PGD for medical sex selection
Chromosomes also determine our sex with females having two X-chromosomes and males having one X and one Y chromosome. There are some serious diseases that particularly affect one sex more than the other for example haemophilia and muscular dystrophy which affect boys. PGD can be used to identify female embryos in such circumstances although, be cause of the way that these conditions are inherited, 50% of these female embryos will be carriers of the condition and at high risk of passing the disease on to their sons. For this reason it is often preferable to perform single gene PGD (see point 4 below) for conditions like haemophilia and muscular dystrophy so that healthy, non-carrier embryos of either sex can be identified. There are other conditions where sex selection by PGD can be used to minimise (but not avoid) the chance of a child being affected. An example of this is families who already have a child affected with autism. Their risk of having a second autistic child is about four fold higher if that child is a boy. And so some of these couples access PGD and sex selection to reduce their risk of having an autistic child. Different states in Australia have different laws and regulations surrounding sex selection of embryos and currently this type of PGD is only available for medical reasons.
4. PGD for serious genetic disease
Human DNA is made up of more than 20,000 specialised sequences called genes, each of which has a particular function. An error, or mutation, in just a single gene may result in a serious disease such as cystic fibrosis or thalassaemia. Some genetic diseases can only be inherited by offspring if both parents carry a mutation in the same gene. In other cases, just one parent with a mutation is enough to put children at high risk of inheriting the disease. PGD can be used by these couples to identify which of their embryos are free of the serious disease that affects their family.
5. PGD for tissue matching
There are some serious childhood diseases, such as certain types of leukaemia or thalassaemia, that can be effectively treated using blood products from a healthy donor. Finding a donor that has matching tissue which will not be rejected can be extremely difficult. Rarely, some couples who have a child with this type of illness, have used PGD to have another child who will be a tissue-match for the sick sibling. At the birth of the baby who resulted from PGD, the umbilical cord is not discarded, but stored so that its blood cells can be used to treat the sick older child.
This type of PGD, sometimes called HLA typing or saviour sibling PGD, has been controversial and different restrictions are placed on its application in different jurisdictions within Australia.
Technology used in PGD
PGD is a very specialised area of genetic testing. The type of genetic testing that is done is dependent on the specific circumstances. Some tests look at chromosomes whilst other more specific tests look at the actual DNA code.
1. Chromosome testing
For almost 20 years chromosome testing in PGD was achieved using fluorescent in situ hybridisation (FISH), where DNA probes labelled with a coloured fluorescent tag were used to analyse up to ten different chromosomes in each cell. This approach was limited because not all chromosomes could be tested.
Technological developments in genetic testing have advanced rapidly over recent years. It is now possible, using a number of different approaches, to rapidly and accurately test all chromosomes even in a single cell.
Array comparative genomic hybridisation (aCGH):
- aCGH systems analyse at least 3000 different sites across all of the chromosomes by comparing the DNA from the embryo to known normal DNA. aCGH systems are highly accurate and rapid and provide just the right amount of information to determine whether there is any aneuploidy present
- More than 90 per cent of advanced PGD laboratories in the world currently use aCGH for aneuploidy testing and this is currently the technique of choice
- Can be used in PGD for analysis of aneuploidy, chromosome rearrangements and translocations and gender
Single nucleotide polymorphism (SNP) arrays :
- SNParrays can potentially provide information on hundreds of thousands of different sites across all chromosomes. This is achieved by exploiting the fact that we all have small variations, or poly morphisms, in our DNA. Most of these variations are normal and have no impact on our health, but they can be used to determine the number of copies of individual chromosomes that are present
- Can be used in PGD for analysis of aneuploidy and gender
Next generation sequencing (NGS):
- NGS is the most recent development in detecting aneuploidy in cells from embryos. NGS can determine the sequence of short stretches of a very small proportion of the total DNA. However, these short stretches are scattered across all chromosomes and in this way NGS can determine how many copies of each chromosome are present
- The use of NGS technology in PGD is increasing. NGS provides about the same amount of information and probably has the same degree of accuracy as aCGH but may prove to be more cost effective in the future
- Can be used in PGD for analysis of aneuploidy, chromosome rearrangements and translocations and gender
2. Genetic testing for serious disease
Multiplex-PCR
Most severe genetic diseases are caused by mutations or errors in just one gene which is comprised of a very small segment of DNA. Sometimes just one ‘letter’ of the genetic code can be wrong. So the tests to detect these errors must be targeted specifically at that gene. As well as detecting the mutation it is necessary to use linked markers which are on the same piece of DNA as the gene being tested, and are inherited alongside the gene. This approach utilises a technique called multiplex-PCR (polymerase chain reaction), which provides a very accurate and robust method of identifying the mutation. The linked markers are specific to each individual person, somewhat like a DNA fingerprint, and so a special customised test must be developed for each couple requesting PGD. This can take some weeks, depending on the complexity of the test. It is important to under stand that multiplex-PCR only investigates the genes involved in the disease, it cannot provide information about aneuploidies or errors of other chromosomes.
Multiplex-PCR PGD tests have been developed for several hundred different genetic diseases by laboratories throughout the world. Some of the conditions to which PGD has been more commonly applied are listed below.
- Cystic fibrosis
- Huntington disease
- ß-thalassaemia
- Fragile X syndrome
- Spinal muscular atrophy
- Duchenne muscular dystrophy
The multiplex-PCR approach is amenable to almost all genetic conditions, meaning that a PGD test can probably be developed even for those conditions that are very rare or have never been subjected to PGD before.
Karyomapping
Karyomapping is a novel and exciting technology that has recently been introduced in advanced PGD laboratories. Karyomapping uses SNP technology to determine which segments of DNA an embryo has inherited from the parents, i.e. the segment with the normal gene or the mutated gene. Karyomapping is a universal test that can be readily applied to most genetic diseases and is appropriate for more than 90 per cent of patients who require PGD for serious genetic disease. Specific individualised tests do not have to be developed which saves patients time and money. Moreover, karyomapping is able to simultaneously detect aneuploidies and other chromosome errors which should improve pregnancy rates and reduce miscarriages.
Obtaining a sample for testing: embryo biopsy
The first stage in any PGD analysis is to obtain a sample from the embryo for testing, this is called an embryo biopsy. The three main ways of achieving this are polar body biopsy where the embryo is tested at the 1-cell stage, cleavage stage biopsy where one cell is removed at the six to eight cell stage and blastocyst biopsy where multiple cells are removed on day five or six of development. Each of these approaches has advantages and disadvantages.
1. Polar body biopsy
The first and second polar bodies are vestigial packages of DNA that are extruded from eggs during the processes of maturation and fertilisation. Polar body biopsy has been successfully used for PGD for many years. One advantage of polar body biopsy is that it samples biological material that will never become part of the developing embryo. However, there are a number of significant disadvantages. In particular, it can only detect genetic errors that have arisen from the mother, not the father. Also, separate testing needs to be performed on both the first and second polar bodies for each egg/embryo making it an expensive approach. Currently polar body biopsy tends to be practised in countries where later stages of embryo biopsy are prohibited.
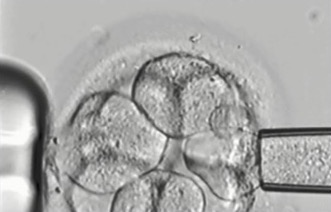
2. Cleavage stage biopsy
Cleavage stage biopsy is performed on day three of embryo development when the embryos have about six to eight cells. A laser is used to puncture a hole in the zona pellucida, the shell-like structure that surrounds each embryo, and then a fine glass pipette is used to gently withdraw one or two cells from the embryo (see fig 1A). The vast majority of PGD cycles per formed throughout the world have been done using cleavage stage biopsy.
Some of the disadvantages of cleavage stage biopsy could be that a relatively large amount of material, approximately 1/8th, is taken from each embryo. The effect of this is not really known although it is likely to be minimal given that identical twins result from a single embryo splitting in half. Testing of just one cell is particularly challenging although experienced PGD laboratories are able to obtain results on about 95 per cent of all embryos after cleavage stage biopsy. Up until recently it was thought that chromosomal mosaicism was particularly prevalent in cleavage stage embryos and that it affected the reliability of PGD results. Recent research using the latest arrayCGH technology has demonstrated that chromosomal mosaicism is low in cleavage stage embryos.
The key advantage of cleavage stage embryo biopsy is that it allows the maximum number of embryos to be tested in PGD. This is particularly important for the many patients who produce only a small number of embryos or whose embryos do not grow particularly well in the artificial environment of the IVF laboratory. It had been thought that slower growing embryos were not viable and so testing them was not necessary, but an increasing amount of evidence now shows that slower growing embryos can often result in healthy babies, particularly if they have been shown to have the normal number of chromosomes by PGD.
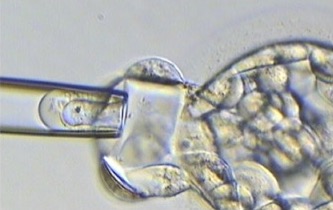
3. Blastocyst stage biopsy
Blastocyst biopsy is performed on day five or six of development when an embryo has formed a fluid-filled ball and may have more than 100 cells. The outside layer of cells is called the trophectoderm, which will go on to form the placenta and other extra-embryonic tissues, and there is an inner clump of cells known as the inner cell mass, which will form the developing foetus. For blastocyst biopsy, a laser is used to puncture a hole in the zona pellucida and then a small number of trophectoderm cells, usually 3-5, are withdrawn from the embryo (fig 1B).
About 50 per cent of all embryos produced in ART laboratories reach the blastocyst stage by day five or six in culture. Consequently, one of the key disadvantages of blastocyst biopsy is that only about half of the embryos can be tested and unfortunately it is quite common that patients will have no embryos at all to test. Some of these slightly slower embryos are -chromosomally normal and could result in a pregnancy, given the opportunity.
There are some potential advantages of blastocyst biopsy. A smaller proportion of the embryo is removed during the biopsy and the trophectoderm sample probably does not include cells that will become the foetus. Success rates appear to be higher than for cleavage stage biopsy, but it must be remembered that blastocyst biopsy is performed on a selected population of the highest quality embryos from patients
Dr Leeanda Wilton
Supported by an untied educational grant from: FERRING
Visit About AccessAustralia at the Our Support tab at access.org.au for more information
Source: AccessAustralia | Australia’s National Infertility Network | www.access.org.au | [email protected]





