This fact sheet describes genetic conditions that are more common in the Ashkenazi Jewish community including the symptoms, causes and any testing which is available.
In summary
∙ Some genetic conditions are more common in the Ashkenazi Jewish community
∙ These include Tay Sachs disease, cystic fibrosis, familial dysautonomia, Canavan disease, glycogen storage disease 1A, mucolipidosis type IV, Niemann-Pick disease, Fanconi anaemia and Bloom syndrome
∙ All these conditions are inherited in an autosomal recessive pattern and genetic carriers for these conditions usually do not have any symptoms.
WHAT GENETIC CONDITIONS ARE MORE COMMON IN THE ASHKENAZI JEWISH COMMUNITY?
From an ethnic or genetic perspective, the Ashkenazi Jewish community is one whose ancestry can be traced to the Jews who settled in Central Europe. Due to various historical factors, including minimal migration, low rates of conversion to Judaism and the community’s propensity to marry only other Ashkenazi Jews, the genetic background of people from the Ashkenazi Jewish community is more similar, or homogeneous, than the general population. Thus, the Ashkenazi Jewish community may be regarded as a separate ethnic group. As with all ethnic groups, there are certain genetic variations that are more common in the Ashkenazi Jewish community than in the general population, and some of these variations may lead to an increase in certain genetic conditions, such as Tay Sachs disease.
Approximately 1 in 7 people from the Ashkenazi Jewish community in Australia will be a genetic carrier for at least one of the genetic conditions listed in Table 42.1. Genetic carriers do not show any signs or symptoms of the condition and are usually healthy.
∙ Being a genetic carrier for these genetic conditions is not like being a carrier of an infectious virus such as hepatitis where the hepatitis virus is carried in the body
∙ Genetic carriers cannot pass it on to others like a virus. They can however, pass the faulty gene on to their children.
There are several theories as to why the frequency of these genetic conditions, and the number of genetic carriers, is high in the Ashkenazi Jewish population.
Historically, the preference for marrying other Jews meant that any genetic variation was contained within the Jewish community. Due to the relatively small Jewish community, there is an increased possibility that a couple have shared ancestors from many generations ago and therefore have a higher chance of being a genetic carrier for the same genetic condition. This is known as the Founder Effect.
Being a genetic carrier for certain conditions more common in the Ashkenazi Jewish community is thought to provide protection for some infectious diseases that would have spread uncontrollably in undesirable conditions like ghettoes, as occurred in Jewish history. For example, being a genetic carrier for Tay Sachs disease is thought to provide some protection against tuberculosis, and being a genetic carrier for cystic fibrosis may provide some protection against cholera. Those who were genetic carriers for these conditions would have had a better chance of surviving infectious diseases and go on to have children.
Table 42.1: A list of genetic conditions that are more common in the Ashkenazi Jewish community
| GENETIC CONDITION | GENE | GENETIC CARRIER FREQUENCY | CHARACTERISTIC SIGNS AND SYMPTOMS |
| Tay Sachs disease | HEXA gene | 1 in 25 | ∙ Affects the central nervous system ∙ Healthy from birth but by 6 months stop smiling, crawling or turning over, lose ability to grasp or reach out, and gradually became blind, paralysed and unaware of their surroundings ∙ Have a ‘cherry-red spot’ on the retina at the back of the eye ∙ Death occurs before 5 years old |
| Cystic fibrosis | CFTR gene | 1 in 25 | ∙ Affects the lungs, pancreas and sweat glands ∙ A build-up of thick, sticky mucus leads to respiratory problems, incomplete digestion and increased salt loss from the sweat glands ∙ With early diagnosis and treatment, 50% of people with CF now live into their late 30s but may have a lower quality of life |
| Familial dysautonomia | IKBKAP gene | 1 in 30 | ∙ Affects the central nervous system ∙ Symptoms include an inappropriate perception of heat and pain, skin blotching, greatly fluctuating blood pressures and problems with digestion ∙ Only a third of patients survive to the age of 20 years. Effective and early treatment is essential to allow survival and independence |
| Canavan disease | ASPA gene | 1 in 40 | ∙ Affects the central nervous system ∙ Healthy from birth, but from 2-4 months lose head control and start having seizures. Gradually lose other abilities such as grasping or reaching out and responding to their surroundings ∙ Head size grows larger than usual ∙ Death usually occurs before 2 years old |
| Glycogen storage disease 1A (von Gierke disease) | G6PD gene | 1 in 40 | ∙ Affects the liver, kidneys and small intestines ∙ A build-up of glycogen may lead to low blood glucose levels causing seizures and distended belly due to liver enlargement ∙ Other symptoms include osteoporosis, hypertension, renal failure, muscle wasting, susceptibility to infections and poor growth and development ∙ With strict dietary management and treatment, life expectancy may be normal |
| Mucolipidosis type IV | MCOLN 1 gene | 1 in 50 | ∙ Affects development and vision ∙ In the first year of life develop delays in motor and cognitive development. Most do not develop speech or walk independently. Visual impairment worsens over time and many will be blind by their teenage years. Also have gastric problems ∙ Usually survive to adulthood, but life expectancy may be shortened |
| Niemann-Pick disease | SMPD1 gene | 1 in 80 | ∙ Affects metabolism ∙ Will develop an enlarged liver and spleen in first few months of life and have poor weight gain. Normal development in first year, then start to progressively lose motor and cognitive skills ∙ All have a ‘cherry-red spot’ on the retina at the back of the eye ∙ Death occurs in early childhood for the more severe form known as Type A |
| Fanconi anaemia | At least 15 genes Most common : FANCA FANCC FANCG | 1 in 100 | ∙ Affects multiple organs in the body ∙ Symptoms include severe anaemia, immune system failure, problems in the growth and development of the arms and legs, darkening of the skin, short stature and kidney problems ∙ A high susceptibility to develop several types of cancer ∙ Diagnosis usually occurs in children between 3 – 12 years old and the average lifespan is between 20-30 years old |
| Bloom syndrome | BLM gene | 1 in 100 | ∙ Affects multiple organs in the body ∙ Symptoms include small body size, hypersensitivity to sunlight, skin patchiness, frequent infections, diabetes, learning disabilities and reduced fertility ∙ A very high susceptibility to develop several types of cancer ∙ Life expectancy may be shortened due to cancer diagnosis. |
WHAT CAUSES GENETIC CONDITIONS THAT ARE MORE COMMON IN THE ASHKENAZI JEWISH COMMUNITY?
Genetic conditions that are more common in the Ashkenazi Jewish community are caused by a variety of genetic changes found in different genes. See Table 42.1 for a list of genes responsible for the more common genetic conditions.
Many of these genes code for enzymes that are important in the functioning of the central nervous system. When there are mutations present in these genes, the corresponding protein is not produced correctly and symptoms of the condition develop.
Our body is made up of millions of cells, and in each cell there are instructions, called genes, that make all the necessary structural components and chemicals for the body to function. These genes are packaged onto little long strands known as chromosomes.
We all have 46 chromosomes arranged into 23 pairs. One copy of each pair is inherited from our mother and the other from our father. The first 22 chromosome pairs are numbered and are known as autosomal chromosomes. The 23rd pair is made up of the sex chromosomes called X and Y. Males have an X and a Y chromosome and females have two copies of the X chromosome.
Since all our chromosomes come in pairs, all our genes also come in pairs. Sometimes, a gene may have a variation in the instruction that causes the gene to no longer function properly. This variation is called a mutation or pathogenic variant, and means that the product produced by the gene, called a protein, is impaired or even absent.
Gene mutations may be inherited from a parent, or occur for the first time in an individual. Once you have a gene mutation however, it may be passed on to future generations. This is referred to as genetic inheritance.
HOW ARE GENETIC CONDITIONS THAT ARE MORE COMMON IN THE ASHKENAZI JEWISH COMMUNITY INHERITED?
The inheritance pattern may vary for different genetic conditions. All the conditions listed in Table 42.1 follow a pattern of autosomal recessive inheritance. Autosomal refers to the fact that the gene involved is located on a numbered chromosome, and therefore affects males and females equally. Recessive means that, in order to develop signs and symptoms of the condition, both copies of the gene must be faulty.
If a couple are both genetic carriers for the same genetic condition (Figure 42.1), in every pregnancy there is:
∙ 1 chance in 4 (25% chance) that they will have a child who inherits both copies of the recessive gene mutation from his/her parents. In this case, no working gene product will be produced and their child will be affected by the genetic condition
∙ 1 chance in 4 (25% chance) that their child will inherit both copies of the working gene and will be unaffected by the condition and not a genetic carrier
∙ 1 chance in 2 (2 chances in 4 or 50% chance) that their child will inherit the recessive gene mutation and the working copy of the gene from the parents and he/she will be an unaffected genetic carrier for the condition, just like the parents.
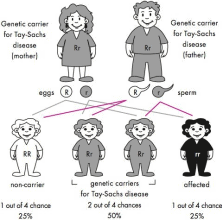
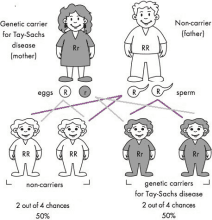
Figure 42.1: Autosomal recessive inheritance where both parents are genetic carriers of the same faulty gene. The faulty gene is represented by ‘r’ ; the working copy by ‘R’.
If only one parent is a genetic carrier
(Figure 42.2) in every pregnancy there is:
∙ No chance that the couple will have a baby affected with the genetic condition caused by this particular gene
∙ 1 chance in 2 (2 chances in 4 or 50% chance) that their child will inherit one copy of the recessive gene mutation and one working copy of the gene from the parents and he/ she will be an unaffected genetic carrier for the condition
∙ 1 chance in 2 (2 chances in 4 or 50% chance) that they will have a child who inherits both copies of the working gene from his/her parents. In this case, the child will be unaffected by the condition
IS THERE ANY TESTING AVAILABLE FOR GENETIC CONDITIONS THAT ARE MORE COMMON IN THE ASHKENAZI JEWISH COMMUNITY?
Diagnostic testing
If your child is suspected of being affected with a genetic condition a series of examinations and blood tests may be arranged by your healthcare practitioner. A referral to a genetics specialist may be appropriate and will be discussed with you.
Figure 42.2: Autosomal recessive inheritance where only one parent is a genetic carrier for a particular genetic condition. The faulty gene is represented by ‘r’; the working copy by ‘R’.
Testing for carrier status
When a person is identified as a genetic carrier for a condition common in the Ashkenazi Jewish community, their first degree relatives (parents, children, brothers and sisters) all have a 1 chance in 2 (50%) of also being a genetic carrier. Genetic testing may be offered to at-risk family members.
Genetic screening is also available for couples from an Ashkenazi Jewish background who are planning a pregnancy to determine their carrier status for the more common genetic conditions, such as those listed in Table 42.1. This is a fast evolving field where the number of conditions being tested for is growing rapidly. It is best to speak with your healthcare practitioner about the availability of genetic screening in your area.
Prenatal testing and PGD
For couples who are both known genetic carriers for a condition common in the Ashkenazi Jewish community, testing may be available during a pregnancy to determine whether the baby will be unaffected, affected or a genetic carrier. It may also be possible to undergo pre-implantation genetic diagnosis (PGD) screening for the genetic condition on an embryo created using in vitro fertilisation (IVF). When possible, these options are best discussed and considered before pregnancy in order to ensure all possible risks, benefits and outcomes can be explored.
www.genetics.edu.au Updated 23 November 2015





